 |
Spin-Coupling/Splitting
Use the Back Arrow to return to a spectroscopy problem
 |
Proton NMR
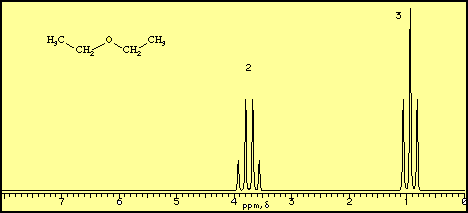
For a molecule such as diethyl ether, CH3CH2OCH2CH3, two types of protons would be predicted to appear in the NMR spectrum; a 'simple' CH3 in the area of 
 1, and a CH2 shifted down to about
1, and a CH2 shifted down to about 
 4 by the electronegative oxygen. The NMR spectrum of diethyl ether, however, displays seven peaks, as shown below.
4 by the electronegative oxygen. The NMR spectrum of diethyl ether, however, displays seven peaks, as shown below.
This multiplicity is due to the phenomena known as spin coupling and arises because of interaction of the proton magnetic field with bonding electrons. In essence, each proton can have one of two possible spin orientations in the applied field, so that the magnetic field sensed by adjacent protons can have one of two possible values. The result is that n protons will split adjacent protons into (n + 1) peaks. The intensities of these peaks are simply a result of the possible spin orientations, thus, the protons of a CH2 group can have the following possible spins:








The middle pair are degenerate, therefore a proton adjacent to a CH2 group will be split into three separate peaks in the ratio 1:2:1.
The separation between these peaks is referred to as the coupling constant, J which is measured in Hz; typical values for J seldom exceed 20 Hz and it is important to note that sets of coupled protons will display exactly the same coupling constant.
In the spectrum for diethyl ether, the CH3 group is split by the two protons on the adjacent CH2 group into three peaks (a triplet) and the absorbance for the CH2 is split by the three protons on the methyl group into (n + 1) = 4 peaks (a quartet). The two ethyl groups in diethyl ether show as a single absorbance since the molecule has a plane of symmetry, that is, both ethyl groups are chemically and magnetically equivalent.
The NMR spectrum of methyl isopropyl ether (CH3-CH(CH3)2) would be predicted to have three types of protons; the methyl group bonded to the oxygen, shifted to 
 4, a methyne proton (the CH), also shifted to the same region, and the isopropyl methyl protons, in a fairly 'simple' environment at
4, a methyne proton (the CH), also shifted to the same region, and the isopropyl methyl protons, in a fairly 'simple' environment at 
 1. The CH3 bonded to the oxygen should appear as a single peak (a singlet) since it is not immediately adjacent to any protons. The CH should be split into seven peaks since it is adjacent to six equivalent hydrogens (the two CH3 groups) and the peak for the two (magnetically equivalent) CH3 groups should be split into two peaks (a doublet) by the adjacent CH. These predictions are consistent with the spectrum for methyl isopropyl ether shown below:
1. The CH3 bonded to the oxygen should appear as a single peak (a singlet) since it is not immediately adjacent to any protons. The CH should be split into seven peaks since it is adjacent to six equivalent hydrogens (the two CH3 groups) and the peak for the two (magnetically equivalent) CH3 groups should be split into two peaks (a doublet) by the adjacent CH. These predictions are consistent with the spectrum for methyl isopropyl ether shown below:

The effects of splitting are often described using a splitting tree which depicts the original absorbance being split by a coupling constant J into (n + 1) peaks.

A splitting tree such as this is useful in understanding more complex splitting patterns, such as those that occur in Br-CH2CH2CH2-OD, as shown below:

As expected, the deuterium (2H) with spin = 1, does not show in the proton NMR. The methylene group adjacent to the bromine is shifted to  3.4 and the methylene adjacent to the oxygen is shifted to
3.4 and the methylene adjacent to the oxygen is shifted to  3.75; both of these appear as simple triplets, each split by the central CH2. The coupling constants for these splitting patterns are slightly different; the ab constant is J = 15 Hz and the bc constant is J = 12 Hz (there is no simple way to predict this ahead of time). The splitting pattern for the central methylene is more complex, being split by the protons on carbon a into a triplet, J = 15 Hz, and each of these peaks being further split by the protons on carbon c into triplets with J = 12 Hz.
3.75; both of these appear as simple triplets, each split by the central CH2. The coupling constants for these splitting patterns are slightly different; the ab constant is J = 15 Hz and the bc constant is J = 12 Hz (there is no simple way to predict this ahead of time). The splitting pattern for the central methylene is more complex, being split by the protons on carbon a into a triplet, J = 15 Hz, and each of these peaks being further split by the protons on carbon c into triplets with J = 12 Hz.

As shown by the splitting tree, the spectrum is predicted to consist of 9 peaks centered around  1.53. The actual NMR, as shown above, shows only five peaks in this region, This is because the central seven peaks are only separated by
1.53. The actual NMR, as shown above, shows only five peaks in this region, This is because the central seven peaks are only separated by  2 Hz, and the 60 MHz spectrometer cannot resolve the individual peaks. Higher resolution spectrometers (i.e., 600 MHz) are useful at resolving fine structure in the spectrum such as this.
2 Hz, and the 60 MHz spectrometer cannot resolve the individual peaks. Higher resolution spectrometers (i.e., 600 MHz) are useful at resolving fine structure in the spectrum such as this.
13C NMR
Spin coupling, while possible between adjacent 13C nuclei, is not typically observed since the natural abundance of 13C is very low (1.1%), making it unlikely that two 13C nuclei will reside next to each other in a given molecule. Attached protons, however, with a spin of 1/2, will couple with the 13C nucleus to generate spin-coupling. As described above, the signal from the carbon will be split into (n + 1) peaks, where n is the number of attached protons.
Typically, however, the spectrometer is set up in a mode referred to as proton noise-decoupling in which the sample is irradiated at a second frequency which promotes all of the protons in the molecule to high spin states, disallowing the spin-coupling process. All of the split 13C peaks are thereby reduced to sharp singlets. While this operating mode provides less information than the non-decoupled mode, it is commonly used because it results in a significant signal enhancement due to a phenomena known as the "Nuclear Overhauser Effect. While the details of this process are beyond the scope of this simple tutorial, the important fact to note is that the NOE results in the transfer of energy to attached 13C nuclei, resulting in a significant enhancement in the NMR signal. Unfortunately, the NOE enhancement is not uniform, hence the integration of a 13C NMR spectrum is typically meaningless. In the examples given in this tutorial, we will provide coupling information (singlet, doublet, etc.) along with the chemical shift, but without intensity data.
 H Splitting Patterns
H Splitting Patterns