Research
Salamanders, sea urchins and sea squirts, oh my! Twofold, my research focuses on a symbiotic relationship between unicellular green algae and amphibian egg masses and the regulation of marine invertebrate metamorphosis. Most work in my lab is pure basic research (done for the love of discovery) and I am also a member of the St. FX Centre for Biofouling Research, which is investigating environmentally friendly solutions to marine biofouling (trying to help). Research in the Bishop lab involves summer research assistants, Honours students, Masters students and post-doctoral fellows. See below for more information on work in progress.
| Publications |
Algal salamander symbiosis: better together.
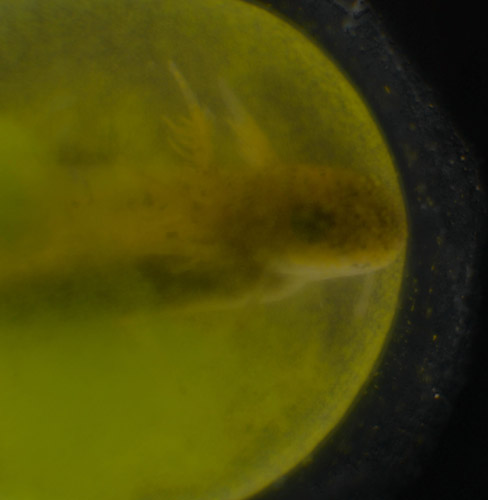
Stage 43 A. maculatum embryo with associated green algae.
Egg masses of several North American amphibians form an association with unicellular green algae. While in Brian Hall's lab at Dalhousie University, I became involved in a project with Ryan Kerney and several others, in which we documented for the first time an intimate association of unicellular green algae with embryos of the northeast yellow spotted salamander Ambystoma maculatum. While an ecto-symbiotic relationship had been recognized for well over a century, we were able to demonstrate that the algal cells were invading tissues and even individual cells of the embryo. The discovery of this novel symbiosis now opens new avenues of research, with many basic questions about the nature of this interaction to be answered. Currently, we are are using phylogenetics and experimental methods to investigate the identity of algae associated with A. maculatum and several other amphibian taxa. Current research suggests that a single clade of oophilid algae associate with egg masses of four amphibian taxa, indicating that it is an ecological specialist. In collaboration with Dr. Tony Miller, we have discovered that the symbiont possesses a remarkable capacity to quench full sunlight and are currently investigating the mechanism underlying this pattern of non-photochemical quenching. Dr. Daniel Small is employing this symbiosis to test oxygen-limitation hypotheses of thermal tolerance.

Functional morphology and evolution of sea urchin larvae.
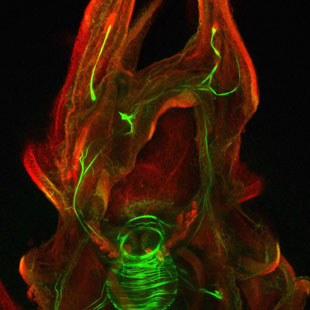
Image Caption
There is wonderful variation in the highly convoluted morphology of sea urchin larvae, but little understanding of how this variation relates to performance. Many organisms reproduce by means of a complex life cycle in which embryos first develop into larvae before transforming through a process called metamorphosis (for animals) into a juvenile, which is the reproductive stage of the life cycle. I have long been interested in cellular and molecular mechanisms that orchestrate metamorphoses of marine invertebrates. This transformation is particularly interesting because it often requires a cue from the future habitat of the adult, invoking ecology, sensory biology, behaviour and developmental plasticity as relevant aspects of studies on metamorphosis. For many years my focus has been on a nitric oxide based signaling system that, for most taxa studies acts to inhibit the onset of metamorphosis. It is currently the most phylogenetically widespread signaling system that regulates metamorphosis, indicating a clear case of homoplasy. Several years ago, I characterized a novel function for a neurogenic region of the post-oral ciliary band, and have since become interested in its distribution in the echinoids. Katelyn MacNeil, a newly graduated Masters student has been working with the "primitive" sea urchin Eucidaris tribuloides to examine the origin and evolution of the canonical sea urchin apical sensory organ and has discovered some interesting things along the way, such as larval arm flapping!

Environmentally friendly anti-fouling solutions.
All marine installations (docks, ships, oil rigs, aquaculture infrastructure etc.) will rapidly become covered with a variety of algae and encrusting animals such as barnacles, tunicates, calcareous worms, mussels and bryozoans. The reason they become encrusted is because these organisms have a life history that involves a freely dispersing propagule (e.g., spore or a larva) that can colonize new habitat. These biofouling species are problematic for a variety of reasons, ranging from economic to ecological, depending upon the circumstance. With a relatively recent ban on eß ffective anti-fouling paints containing tin or copper, the world is now left with very few effective solutions that are environmentally friendly, cost-effective and long lasting. The StFX Centre for Biofouling Reseach is focused upon developing such environmentally friendly solutions. Currently we are interested in the vase tunicate as our model encrusting organism because the local mussel aquaculture industry is beleaguered with this invasive and difficult to eradicate pest. We are working with the Department of FIsheries and Oceans to further understand the population ecology of this species, with the expectation that we will be able to advise farmers how to choose future aquaculture sites, and to mitigate or prevent the spread of the vase tunicate. The grand goal is to design, manufacture and test materials that when submerged in the ocean, prevent the accumulation of encrusting organisms, or at least improve our ability to scrape or wash them off. Additional challenges are to produce such anti-fouling surfaces that are environmentally friendly and inexpensive to manufacture and deploy. This work is ongoing as part of the St. FX Centre for Biofouling Research.

Email us for more information and to request papers
Lin Y and Bishop CD (2015) Identification of free-living Oophila amblystomatis Lambert from salamander and wood frog breeding habitat. Phycologia 52: 183-191.
Small DP, Bennett RS, Bishop CD (2014) The roles of oxygen and ammonia in the symbiotic relationship between the spotted salamander Ambystoma maculatum and the green alga Oophila amblystomatis during embryonic development. Symbiosis 63: 3.
Heyland A, Hodin J, Bishop CD (2014) Manipulation of Developing Juvenile Structures in Purple Sea Urchins (Strongylocentrotus purpuratus) by Morpholino Injection into Late Stage Larvae. PLoS ONE 9: 12: e113866.
Bishop CD, Miller AG (2014) Dynamics of the growth, life history transformation and photosynthetic capacity of Oophila amblystomatis (Chlorophyceae), a green algal symbiont associated with embryos of the northeastern yellow spotted salamander Ambystoma maculatum (Amphibia). Symbiosis 63: 2: 47-57.
Eunsoo Kim, Yuan Lin, Ryan Kerney, Lili Blumenberg, Cory Bishop (2014) Phylogenetic Analysis of Algal Symbionts Associated with Four North American Amphibian Egg Masses. PLoS ONE 9: 11: e108915
Bishop CD, MacNeil KEA, Patel, D, Taylor, VJ, and Burke, RD (2013) Neural Development in Eucidaris tribuloides and the Evolutionary History of the Echinoid Larval Nervous System. Developmental Biology 377: 236–244.
Romero MR, Phuong M, Bishop CD and Krug PJ (2012) Nitric oxide signaling differentially affects habitat choice by two larval morphs of the sea slug Alderia willowi: mechanistic insight into evolutionary transitions in dispersal strategies. Journal of Experimental Biology 216: 1114-1125.
Cameron CB and Bishop CD (2012) Biomineral ultrastructure, elemental constitution and genomic analysis of biomineralization related proteins in hemichordates. Proceedings of the Royal Society B 279: 1740 3041-3048.
Kerney RK, Kim E, Hangarter RP, Heiss AA, Bishop CD, Hall BK (2011) Intracellular invasion of green algae in a salamander host. PNAS 108: 6497-6502.
Bishop CD, Galway ME and Garbary DJ (2010) Architecture and design among plants and animals: convergent and divergent developmental mechanisms. In: Origins and Design in Nature Seckbach J. (Ed.) Springer, NY.
Bishop CD, Bates, WR and Hall, BK. (2010) Heat shock protein 90 expression in two migratory cell types of ascidian embryos and larvae: test cells deposit HSP90 on the larval tunic. International Journal of Developmental Biology 54: 1337-1346.
Bishop CD and Hall BK (2009) Sniffing out new data and hypotheses on the form, function and evolution of the echinopluteus post-oral vibratile lobe. Biological Bulletin 216: 307-321.
Bishop CD, Pires A, Boudko DY, Moroz LL and Hadfield MG (2008) Analysis of NO-cGMP signaling during metamorphosis of the nudibranch Phestilla sibogae (Gastropoda: Opisthobranchia). Evolution and Development 10: 288-299.
Bishop CD and Burke RD (2007) Ontogeny of the holothurian larval nervous system: evolution of larval forms. Development Genes and Evolution 217: 585-592.
Bishop CD and Brandhorst BP (2007) Development of nitric oxide synthase-defined neurons in the sea urchin larval ciliary band and evidence for a chemosensory function during metamorphosis. Developmental Dynamics 236:1535-1546 (A 'Highlighted' article by the journal Sept. 2007).
Bishop CD, Huggett MJ, Heyland AH, Hodin JH, Brandhorst BP (2006) Interspecific variation in metamorphic competence in marine invertebrates: the significance for comparative investigations of regulatory systems. Integrative and Comparative Biology 662-682.
Bishop CD, D. F. Erezyilmaz, T. Flatt, C. D. Georgiou, M. G. Hadfield, A. Heyland, J. Hodin, M. W. Jacobs, S. A. Maslakova, A. Pires, A. M. Reitzel, S. Santagata, K. Tanaka, and J. H. Youson. (2006) What is metamorphosis? Integrative and Comparative Biology 1-5.
Bishop CD and Brandhorst BP. (2003) On NO signaling, metamorphosis and the origin of biphasic life cycles. Evolution and Development 5: 542-550.
Bishop CD, Bates WR and Brandhorst BP. (2002) HSP90 function is required for morphogenesis in ascidian and echinoid embryos. Development Genes and Evolution 212: 70-80.
Bishop CD and Brandhorst BP. (2001) NO/cGMP signaling and HSP90 activity represses metamorphosis in the sea urchin Lytechinus pictus. Biological Bulletin 201:394-404.
Bishop CD, Bates WR and Brandhorst BP (2001) Regulation of metamorphosis in ascidians involves NO/cGMP signaling and HSP90. Journal of Experimental Zoology 289: 374-384.
Bates WR and Bishop CD (1996) Localization of constitutive heat shock proteins in developing ascidians. Development Growth and Differentiation 38: 307-314.
Book Chapters and Opinion Pieces
Small DP and Bishop CD (in press) Algal/animal symbioses: Developmental and phylogenetic patterns. (Solicited book chapter for Algal Symbioses; Joseph Seckbach, Ed.).
Bishop CD, Galway ME and Garbary DJ (2011) Architecture and design among plants and animals: convergent and divergent developmental mechanisms. In: Origins and Design in Nature. Seckbach J. (Ed.) Springer, NY.
Hodin J, Bishop CD, Sharpe F, and Valas R (2009) A creative celebration of evolution. Nature 461(8): 733. (An Opinion piece included as part of the “Darwin 200” celebration).