Chem421 Fall 2011
ASSIGNMENT #3 Due to November 21
- {10} The two triplet diradicals (two weakly coupled unpaired electrons) below have different properties. Structure 1 is a triplet diradical at high temperature but becomes a closed-shell singlet at low temperature. Structure 2 has a triplet electronic state at any temperature. Rationalize these results on the basis of the structures of two diradicals.
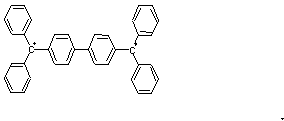

1 2
2. {30} Consider the following Markovnikov addition:
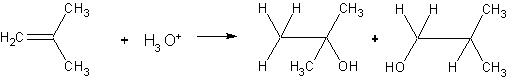
Major Minor
(a) For both products, predict reaction mechanism and reactive intermediates.
(b) Draw the Newman projections for what you think would be the lowest energy conformers for the major and minor products.
(c) Is the reaction under kinetic or thermodynamic control? Explain briefly.
3. {15} Using the experimental ΔHf0 values given below (kcal/mol) and group increment table calculate the strain energy for each compound. Briefly discuss whether the trend seen is consistent with expectations based on additivity of ring strain.
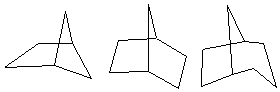
16.37 -12.42 -23.04
- {5} Sketch a Newman projection of what you think would be the preferred conformation of hydrazine (H2NNH2), and briefly explain your choice.
- {5} Predict the preferred conformation of fluoromethanol, FCH2OH, around the C-O bond and briefly rationalize your choice.
6. {10} Convince yourself that at T=5K a 1 kcal/mol barrier is insurmountable.
Use A=1013 s-1 typical of unimolecular reactions.
7. {5} Explain why the structure below readily forms dianion.
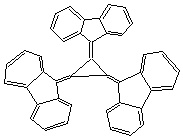
tris-(9-fluorenylidene)cyclopropane
8. {10}
Complete the drawings below to create 10 aromatic systems using (4n+2)
rule.
For example:
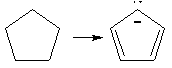
6 π-e- aromatic
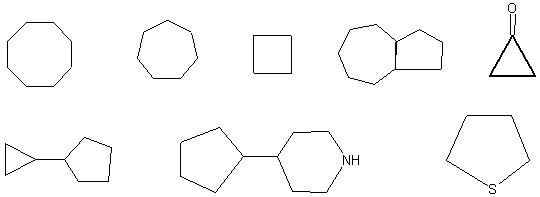
9. {20} Using
Gaussian03 predict the structure (structures) of the ethyl carbenium ion, C2H5+,
with the B3LYP/6-31+G(d) method. Report geometries, Eel and H0.
Does the structure have symmetry?