CHEM_100/11 2012
ASSIGNMENT #3
Due to November 9
GAS LAWS and Chemical equilibrium
1. {4} The atmospheric pressure today in Antigonish is 740.0 mmHg.
(a) {2} What is the pressure in kPa?
(b) {2} What is the pressure in atm?
Solution:
(a)
PkPa =740.0 mmHg
x 101.325kPa/760 mmHg
= 98.65855 kPa = 98.66 kPa
(b)
Patm = 740.0 mmHg
x 1 atm/760 mmHg = 0.9737 atm
2. {6} Calculate the pressure (in atm) of a sample of O2 gas (12.0g) in a steel vessel of 15.0 L at 18.0 oC.
PV=nRT; P=
nRT/V
mol O2 :
12.0 g x 1mol/32.0g = 0.375 mol
R = 0.0821 L atm/mol K
T= 18 + 273 =291K
P = (0.375 mol x 0.0821 L atm/mol K x 291 K) / 15.0L
=0.597 atm
3. {5} A propellant in an aerosol spray has a pressure of 5.00 atm at 16o C. Instruction on the can includes “do not expose to heat above 60o C or explosion may result”. What is the pressure inside the can at 61oC?
Solution :
P1V1/n1T1 = P2V2/n2T2
= R (constant) ;
since V and n are constant, P1/T1 = P2/T2 P2 = P1 T2/T1
T1 = 16 +273 = 289 K
T2 = 61 + 273 = 334 K
P2 = 5.00 atm x 334 K/ 289 K = 5.8 atm
4. {10} You synthesized a greenish-yellow gaseous compound of chlorine and oxygen and found that its density, d, is 7.71 g/L at 36oC and 2.88 atm.
(a) {5} Calculate the molar mass, M.
(b) {5} Using molar mass and atomic masses of Cl and O, determine the molecular formula by trial and error.
(a) PV=nRT; n/V= P/RT; nM/V=
MP/RT; d= MP/RT; M = dRT/P
M= 7.71 g/L (0.0821 L atm/K mol) (36+273)K/2.88atm =
67.9 g/mol
(b) Cl- 35.45 g, O-
16.00g
One Cl + one O-
51.45 (too low);
2 Cl +1O = 86.9 –
too high
1Cl +2O = 67.45 - the formula should be ClO2.
5. {10} It takes 68.3 s for a sample of N2 (g) to effuse through a tiny hole. Determine the molecular mass of gas whose effusion time under exactly the same conditions is 85.6s.
(remember that rate and time are inversely proportional)
r1/r2
= √ M2/M1 = t2/t1
; t22/t12
= M2/M1
a) 68.32 s2/85.62s2
= 28 g/mol/M1
= 0.637 ; M1 = 28g/mol / 0.637 = 44 g/mol (probably, CO2)
7. {10} Write the expressions for Kc for the following reactions. In each case indicate whether the rxn is homogeneous or heterogeneous.
(a) N2 (g) + O2 (g) à 2NO (g)
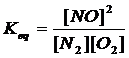 homogen
homogen
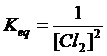 (b) Mo (s)
+ 2Cl2 (g) à MoCl4 (l)
(b) Mo (s)
+ 2Cl2 (g) à MoCl4 (l)
heterogen
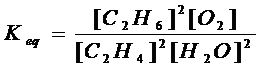 (c) 2C2H4
(g) + 2H2O (g) à
2 C2H6 (g) + O2 (g)
(c) 2C2H4
(g) + 2H2O (g) à
2 C2H6 (g) + O2 (g)
homogen
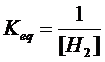 (d) FeO (s)
+ H2 (g) à Fe (s) + H2O (l)
(d) FeO (s)
+ H2 (g) à Fe (s) + H2O (l)
heterogen
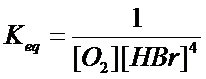 (e) 4HBr
(aq) +
O2 (g) à H2O (l)
+ 2Br2 (l)
(e) 4HBr
(aq) +
O2 (g) à H2O (l)
+ 2Br2 (l)
![]() heterogen
heterogen
8 {10} Determine the value of Keq for the reaction of sodium and carbon dioxide in water using the data given below
2Na(s) + 2H2O(l) + 2CO2(g) D 2NaHCO3 (s) + H2 (g)
Na (s) + H2O (l) D NaOH (aq) + ½ H2 (g) Keq = 7.3 x 1031
Na2CO3 (s) + H2O (l) D 2NaOH (aq) + CO2 (g) Keq = 6.6 x 10-9
2NaHCO3 (s) D Na2CO3 (s) + H2O (l) + CO2 (g) Keq = 1.1 x 10-5
Solution:
2Na (s) +
2H2O (l) D
2NaOH (aq) +
H2 (g) Keq = (7.3 x 1031)2
+
Na2CO3
(s) + H2O (l) + CO2 (g) D2NaHCO3 (s)
Keq
= (1.1 x 10-5)-1
+
2NaOH (aq) + CO2 (g) D Na2CO3 (s) + H2O (l) Keq
= (6.6 x 10-9)-1
____________________________________________________________
2Na(s) + 2H2O(l) + 2CO2(g)
D
2NaHCO3 (s) + H2 (g)
Keq = (7.3 x 1031)2 x Keq
= (1.1 x 10-5)-1 x (6.6 x
10-9)-1 = 7.3 x 1076
9. {10} At 22 oC, KP = 0.070 for the equilibrium
NH4HS (s) à NH3 (g) + H2S (g)
A sample of solid NH4HS is placed in a closed vessel and allowed to equilibrate.
(a) {8} Calculate the equilibrium partial pressure (atm) of ammonia, assuming that some solid NH4HS remains.
b) {2} What is the total pressure in the vessel at equilibrium?
a) Kp = PNH3 x PH2S PNH3 = PH2S PNH3 = √Kp
PNH3 = √ 0.070 = 0.26 atm
b) PNH3 = PH2S Pt = PNH3 + PH2S
P t = 0.26 + 0.26 =0.52 atm
10. {10} Consider the following reaction at equilibrium:
2CO2 (g) à 2CO (g) + O2 (g) ΔH = -514 kJ
How do the following changes affect the amount of CO(g) ?
(a) increase temperature decrease
(b) more CO2 added increase
(c) more O2 added decrease
(c) volume of the vessel is tripled incr.
(d) O2 is removed incr.
(e) Ar gas is added no effect
(f) total pressure is increased decr.
(g) a catalyst is added no effect
(h) remove some CO2 (g) decr.
(i) decrease temperature incr.
11 {5} For the equilibrium
N2 (g) + 3 H2 (g) D 2NH3 (g)
the Kp = 4.51 x10-5 at 450 oC.
For the mixture below indicate the direction of this equilibrium reaction (toward product or toward reactants) at the following partial pressures:
105 atm NH3; 35 atm N2 ; 495 atm H2
Q = (105)2/(495)3 x 35
= 2.6 x 10-6
Q<Keq therefore rxn
will shift to the product.
- {10} at 1000C, Kc = 0.078 for the rxn:
SO2Cl2 (g) D SO2 (g) + Cl2 (g)
At the equilibrium, the concentrations of SO2Cl2 and SO2 are 0.108 M and 0.052 M, respectively. What is the partial pressure of Cl2 at equilibrium? (hint: modify the ideal gas equation using the fact that n/V=M )
Solution:
Kc = [SO2]
[Cl2]/[SO2Cl2] à [Cl2] = Kc [[SO2Cl2]/ [SO2]
= 0.078x0.108/0.052= 0.16M
PV = nRT; PV/V = (n/V) RT; P= MRT
P = 0.16
mol/L x 0.0821 L atm mol-1K-1
373 K = 4.9 atm