CHEM_100_11 2013
Assignment #5
KINETICS
(chapter 14)
1.
{14} Indicate the order ( zero, 1st or 2nd)
of the rxn Aà B for each of the following observations
a)
A plot of the inverse of the concentration
[A] versus time yields a straight line (2nd)
b)
The unit of rate constant is Ms-1 (zero)
c)
The rxn has a half-life that is independent of the initial
concentration, [A]0 (1st order)
d)
the concentration of reactant [A] fall to
one-half in equal intervals of time (1st order)
e)
the half-life of the rxn gets shorter as
the initial concentration is increased (2nd order)
f)
A
plot of the ln [A]
versus time yields a straight line (1st order)
g)
When the initial concentration [A] doubles,
the rate doubles (1st order).
h)
When [A] is reduced by a factor of 2, the
rate of the reaction is reduced by a factor of 4 (2nd
order).
i)
Change
in [A] does not change the rate (zero)
j)
the unit of the rate constant is M-1s-1
2d order
k)
the unit of the rate constant is s-1
1st
order
l)
the rate law
is described by the formula : rate=k[A]2
second order
m) A plot of [A]
versus time yields a straight line. (zero)
n)
The rate law is
described by the formula rate=k (zero)
2.
{10} The data in the table below were
obtained using the initial rate method for the reaction:
2ClO2 (aq)
+ 2OH- (aq) à
ClO3- (aq) + ClO2-(aq) + H2O (l)
exp. number [ClO2],
M [
1 0.060 0.030 0.0248
2 0.020 0.030 0.00276
3 0.020 0.090 0.00828
a)
{5} analyze data and deduce the
differential rate law
b)
{2} calculate the magnitude of the rate
constant
c)
{1} what is the overall order of this
reaction?
d)
{2} calculate the reaction rate for [ClO2]=[OH-]=
0.01M
Solution: (0.060/0.020)n =
(0.0248/0.00276); 3n =9, n=2
(0.09/0.03)m =
(0.00828/0.00276); 3m
= 3, m=1
a)
Reaction is first order
in OH- and second order in ClO2
rate= k [ClO2]2[OH-]
b)
k= rate/([ ClO2]2[OH-])
= 0.0248Ms-1/(0.0602M2 x 0.030 M) = 229.6= 230
M-2s-1
c)
2+1 =3
d)
rate= 230 M-2s-1[0.01]2[[0.01]
= 0.00023 M/s
3. {10}
The first-order rate constant for the reaction of methyl chloride (CH3Cl)
with water to produce methanol (CH3OH) and hydrochloric acid (HCl) is 3.32 x 10-10 s-1 at 25 oC. Calculate the rate constant at 40oC
if the activation energy is 116 kJ/mol.
Solution
T1
= 25+273 = 298K
T2=
40 +273= 313K, k2 = ?
ln (k1/k2) = Ea/R (1/T2-1/T1)
= (116 x 103 J/mol)/(8.314 J/K mol)
[(1/313K-1/298K) = 1.395 x 104 [-0.000161] = -2.244
k1/k2 = e-2.244 = 0.106
k1/k2 =0.106; k2 = (3.32 x 10-10 s-1)/
0.106 = 3.13 x10-9 s-1
4. {10} Consider the first-order reaction Aà
product, with k=2.95 x 10-3 s-1. What percent of A remains after 100 s?
Solution
ln([A]t/[A]0) = -kt = -2.95 x 10-3 s-1 x
100 s-1 = -0.295 ; [A]t/[A]0
= e-0.295 = 0.745
[A]t
= 0.745 [A]0 – the fractional part of A remaining.
0.745 x 100
= 74.5%
5. {10} the reaction 2Aà B is second order with
a rate constant of 52 M-1 min-1 at 25oC.
(a) {5} Starting with [A]0
= 0.0082M, how long will it take for [A]t = 2.7 x 10-7 M?
(b) {5} Calculate the
half-life of this reaction.
Solution
t1/2 =
1/k[A]0 ; 1/[A]t
= kt + 1/[A]0 ;
1/[A]t - 1/[A]0 = kt
= 1/2.7x10-7M - 1/0.0082 M
= 0.37 x 107M-1
– 122.0 M-1 = 3699878 M-1; t = 3699878/52 =71151min=1186hr = 49.4 days
t1/2 =
1/(52x0.0082) = 2.35 min
6. {15} The reaction Aà
2B + C is first order. If the initial [A] =1.50 M and k=1.02 x10-3 s-1,
(a) {10} what is the value of [A] after 400
s?
(b) {5} What is
the concentration of B after the 400 s? Assume that volume is constant during
the rxn.
(a)
ln [A]t = -kt + ln [A]0 ;
ln[A]400
= -1.02 x 10-3 s-1 x 400 s + ln 1.50 = -0.408
+ 0.405 = -0.003;
[A]400
= e-0.003 = 0.997M
(b) [A]
consumed = 1.50M-0.997M = 0.503M
[B] = 2 x 0.503M = 1.01M
7. {5} The reaction 2Aà B is first order in A
with a rate constant of 1.5 x 10-2 s-1 at 100oC.
How long (in seconds) will it take for A to decrease from 0.30M to 0.10 M?
Solution
ln [A]t = -kt + ln [A]0
ln([A]t/[A]0) = -kt
= ln(0.10/0.30) = -1.099; t= 1.099/1.5x10-2
= 73s
8. {6} For the elementary process N2O5 (g) à NO2(g) + NO3(g) the activation energy, Ea, is 154kJ/mol and overall ΔE reaction is 136 kJ/mol.
(a). {2} Sketch the energy profile for this reaction, and label Ea and ΔE.
(b) {2} What is the activation energy for the reverse reaction?
(c) {2} Which reaction is faster, forward or reverse?
Solution:
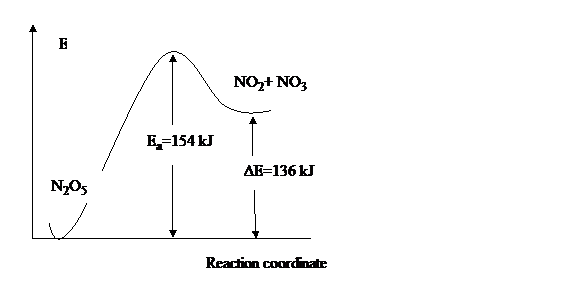
Ea
(reverse) = 154-136= 18 kJ
Reverse
reaction is faster because the activation energy is lower
9. {10} Understanding the high-temperature behavior of nitrogen oxides is essential for controlling pollution generated in automobile engines. The decomposition of NO to N2 and O2 is second order with rate constant of 0.0796 M-1s-1 at 737 0C and 0.0815 M-1s-1 at 947 0C. Calculate the activation energy for the reaction.
Solution:
R= 8.314
J/mol K
T1
=737 + 273 = 1010K
T2=
947 + 273 =1220K
ln(k1/k2)
= Ea/R (1/T2 -1/T1)
ln(0.0796/0.0815) = Ea
/8.314 J/molK(1/1220-1/1010)
Ea
= 8.314(-0.023589)Jmol-1K-1/(-1.704
x 10-4)K-1 = 1.151 x 103 J/mol
= 1.15 kJ/mol