DISCUSSION
A significant feature became apparent on examining the benthic studies of the Atlantic from the last 60 years; there has been a loss of emphasis on this component of the marine ecosystem in recent years. If study effort is allocated as "study-year" (i.e., one study-year = a study occurring in a given year, so a 3 year study is 3 study years), then 62% of the research reported here occurred between 1950 and 1980, with another 20% having taken place between 1930 and 1940. The years 1980 to the present comprise only approximately 16% of the research effort directed at benthic communities since 1930. Further, of the eight studies which make up the effort for the last 18 years, four were directed at other questions (e.g., commercial lobster, scallop issues) and benthos was only included as a secondary component and so in very little detail. In contrast, from 1960-1980 there were 12 published Canadian studies including benthos data, all of which were concerned with benthic ecology, to differing levels of detail, rather than treating the benthic community secondarily to commercial species.
The 42 studies (see Figure 3) reported here range over a large area and diversity of environments, yet they contain a good deal of consistency in results. This consistency may be useful in generalizing to St. Georges Bay, though it must be recognized that this is in no way a substitute for the field sampling of benthic fauna/communities in St. Georges Bay (see Recommendations).
Within environmental conditions (water depth and substrate type) relatively similar to St. Georges Bay the greatest number of individual species per phyla/class (i.e., diversity within phyla/class) appears to belong to the polychaetes. Due to the differences in sampling methods and target species, direct comparison of polychaete diversity between studies is not feasible. However, in those studies which reported polychaete numbers, these invertebrates accounted for between 10 and 70% of the total species present. Within Northumberland Strait, polychaetes represented 26.5-38% of all species present (Table 21). The classes which contributed the next greatest species numbers were the bivalve and the gastropod molluscs. These two groups occurred in generally similar numbers (i.e., between 0.6-3.8 bivalves/gastropod) and together represent approximately 12-37% of the total species present on the substrate. Sanders (1968) reports that the combination of polychaetes and bivalves comprise about 80% of the animals by number within many environments - deep sea, tropical shallow water, tropical estuary, and boreal shallow water. Within the studies reported here, the combination of polychaetes and bivalves ranges generally between 22 and 56% of the total species number, with only one study (Day et al., 1971) reporting species numbers for these two groups at 80% of total species present.
Table 21. Summary of number of species per taxa for various areas reported in Atlantic Canada/U.S.A. Absence of species in a row does not indicate that group is not present, only that it was not samled for/recorded/analyzed.
|
Northumberland Strait
|
Southern Gulf
|
Northern Gulf
|
Fundy
|
|||||||
|
Dunbar et al. (1980)
|
Caddy et al. (1977)a
|
Caddy et al. (1977)b
|
Anonymous (1997, 1998)
|
Hughes & Thomas (1971a)
|
Hughes & Thomas (1971b)
|
Brunel (1971)
|
Peer (1963)
|
Robert (1979)
|
Caddy (1970)
|
|
| Depth (m) Substrate |
5-49
Gravel/coarse material; sand; silt-clay |
7-49
Mud; mud-clay |
5-20
Cobble; shell; sand; silt; bedrock; boulders |
0.3-5.4
|
0-4.7
Silt-clay to coarse sand |
9-100
Sand; muddy sand; mud |
73 & 86
Unsorted gravel to fine sand |
15-150
Silt-sand; silt-clay; sand-silt |
55-128
Rock; gravel; sand; mud |
|
| Bivalves |
23 |
26
|
19
|
8
|
14
|
7
|
11
|
36
|
18
|
|
| Gastropods |
9
|
16
|
5
|
14
|
9
|
6
|
16
|
14
|
||
| Crustaceans |
4
|
11
|
6
|
17
|
12
|
|||||
| Polychaetes |
91
|
58
|
7
|
20
|
7
|
19
|
22
|
17
|
||
| Amphipods |
73
|
41
|
2
|
9
|
||||||
| Echinoderms |
6
|
5
|
3
|
1
|
12
|
4
|
18
|
|||
| Algae |
12 |
74
|
25
|
8
|
||||||
| Total reported species |
N/A
|
343
|
153
|
68
|
62
|
Not reported
|
Not reported
|
31
|
52
|
130
|
a=Entire Northumberland Strait
b=Area D only
|
Buzzards Bay
|
Cape Cod Bay
|
Greenwhich Bay
|
Cape Lookout
|
||
|
Sanders (1958)
|
Sanders (1960)
|
Young & Rhoads (1971)
|
Stickney & Stringer (1957)
|
Day et al. (1971)
|
|
| Depth (m) Substrate |
7-20
Sand; silt-clay |
19
Silt-clay |
12-42
Sand; silt; clayey-silt |
3-9
Silt-mud; mud |
2.5-80
Fine to coarse mud |
| Bivalves |
4-7
|
12
|
18
|
19
|
2-13
|
| Gastropods |
5
|
14
|
6
|
13
|
1-2
|
| Crustaceans |
3
|
3
|
12
|
2-4
|
|
| Polychaetes |
11-17
|
33
|
46
|
37
|
13-50
|
| Amphipods |
4-16
|
21
|
18
|
4
|
4-11
|
| Echinoderms |
1
|
5
|
2
|
1-2
|
|
| Total reported species |
Not reported
|
95
|
113
|
114
|
3-79 (per station)
|
Species presence of non-amphipod crustaceans are variable in their representation, depending upon the location being sampled, but based on the studies reviewed here these crustaceans are present as 3-16% of the total species. Amphipods are not consistently reported, though at some locations (e.g., Northumberland Strait) they obviously form a large contribution to the total species diversity. The echinoderms are consistently represented with low species number, generally <10% of total, and <15% of total species in all reviewed studies. The remainder of the species consist of nematodes, tunicates, hydroids, bryozoans, cnidarians, cumaceans, poriferans, polyplacophorans, and several associated phyla present only in minor quantities. There are indications that the diversity of the benthic fauna is greatest in intermediate depths (e.g., < 75 m depth).
Individual organism densities of >1,000/m2 are not uncommon for some species of bivalves, gastropods, polychaetes and amphipods on the appropriate substrate. In contrast, predator species (e.g., decapod crustaceans and seastars) are almost always reported at < 4/m2. The meiofauna (primarily nematodes) within the substrate, largely ignored in marine benthic studies, are present at densities approaching three orders-of-magnitude greater than the most common macrofaunal species. The density of total fauna (all species combined) commonly exceeds 1,000 organisms/m2, but such assessments are very dependent upon the level of detail of the investigator. There are suggestions that the greatest densities of total individuals (all species combined) occurs in relatively shallow water (<60 m) and decreases in deeper water.
The biomass of benthic organisms fluctuates over the annual season but appears to range from <5 g to as high as 1,400 g wet weight/m2 based on the limited information provided by the reported studies. Unfortunately, biomass is not reported as often as species numbers and densities. In addition, variations in the analysis and reporting of biomass (e.g., wet weight, dry weight, ash free dry weight, with or without shells/tests) make comparison of the limited biomass information impossible. As generalizations, polychaetes, bivalves, gastropods and echinoderms appear to often form the bulk of the invertebrate biomass in northern Atlantic waters, with polychaetes contributing to a greater degree if shells and tests of the other classes/phyla are excluded. Larger but less commonly occurring taxa, such as decapod crustaceans, form only a minor component of the benthic biomass. Often the bulk of the biomass (>95%) is represented by only a few species (i.e., <17).
Deposit and suspension feeders were generally reported to dominate the benthic communities, though other trophic guilds (browsers, carnivores, omnivores, ectoparasites) are also present. Deposit feeders appear to be between 2 and 20 times as abundant as suspension feeders, though this is entirely dependent upon the substrate being sampled. Deposit feeders predominate on silt-clay bottoms while the suspension feeders are more common on sand substrate.
The substrate plays a dominant role in structuring the benthic community and determining what taxa are present. Suspension feeders are most abundant on sandy sediments free from large amounts of silt and clay (Levinton, 1972); a median grain size of 0.18 mm diameter has been theoretically postulated as the optimal size for this trophic guild (Sanders, 1958). The higher velocity currents over sand bottoms, relative to mud bottoms, is thought to assist in suspending and transporting food to suspension feeders (Sanders, 1958). In contrast, deposit feeders are more abundant on finer silt/mud sediments; it has been hypothesized that part of the reason is that the larger surface area of the smaller particles provides more surface for growth of a primary deposit feeder food, bacteria (Levinton, 1972). As well, the slower water currents allow the settling out of food particles (Sanders, 1958) and so an enrichment of the sediment from above.
Probert (1984) indicates that this is not simply passive selection of substrate by the guild, but that once established, the organisms can themselves substantially alter the properties of the sediment. Sanders (1968) suggests that the tubes of amphipod and polychaetes increase sediment stability and spatial complexity of the sand, increasing the diversity due to the greater variety of microhabitats. It has been argued by Gray (1981) that mixed communities of deposit and suspension feeders tend to be the rule rather than distinct communities of each. Thus, while the substrate grain size is correlated with benthic distribution; the mechanisms controlling this distribution and the interactions between various guilds remain unclear.
4.1 IMPLICATIONS FOR ST. GEORGES BAY
The St. Georges Bay study area substrate is composed of distinct areas of gravelly poorly-sorted sands, coarse gravel to well sorted sand, fine sands, very sandy fines, silty mud, sandy mud, and muddy sand, as well as a continuum of combinations of these substrate types. Therefore, it should be expected that there will be a large number and variety of benthic communities within the study area. Figure 5 attempts to capture this by generalizing diversity, trophic guilds, individual phyla/classes and dominant phyla for the various substrates found within St. Georges Bay. This figure indicates changes in these parameters based on differing substrates. For example, bivalves (species numbers, density, biomass) may be expected to increase with decreasing particle size (i.e., sands, silts) down to a point at which the substrate is too fine (i.e., anoxic, difficult burrowing, etc.) when the bivalves may be expected to decrease; only specialized species will be able to inhabit these sediments. It is necessary that it be recognized that this figure is a generalization of studies from elsewhere and that the sediments constitute a continuum of substrates.
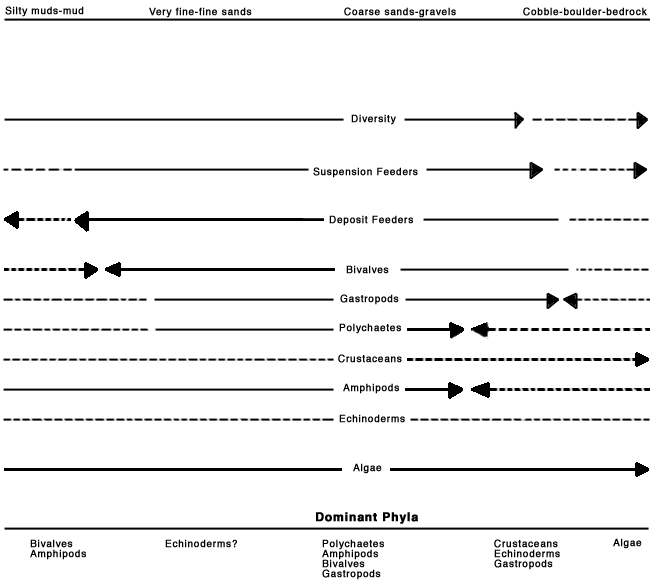
FIGURE 5: Generalized relationships of diversity, trophic guild, and phla to sediment type based on review of studies within this document. Broken lines indicate hypothesized (unknown) trajectories.
Following the philosophy of Jones (1950) that "It is probably true that no two assemblages of animals from different places are ever exactly alike, but it is possible to draw up lists of species that will almost certainly be found on a particular type of bottom within the region, provided that temperature and salinity are within some limits." A preliminary species list (Table 22) has been constructed below based on Northumberland Strait sampling. Due to the commonality of these species among studies, and their abundance, it is suggested that they will also probably form significant components of the benthic community on the appropriate substrate. The low number of polychaetes in Table 22 is more likely a lack of sampling for them than a lack of presence, based on the majority of studies reporting an abundance of species and densities of Polychaeta.
Table 22. Common (abundant or widespread) species from Northumberland Strait studies (Tables 1-3) and hence likely to occur in St. Georges Bay.
|
Algae
|
Bivalves
|
Gastropods
|
Crustaceans
|
| Antithamnion sp. Asperococcus echinatus Ceramium Ceramium fastigatum Ceramium rubrum Chaetomorpha melangonium Chaetomorpha sp. Chondrus crispus Chorda filum Chordaria flagelliformis Cladomorpha sp. Cladophora albida Cladophora seriacea Cladophora sp. Corallina offinalis Cystoclonium ceranoides Enteromorpha Enteromorpha linza Eudesme virescens Euthora cristata Fucus serratus Gelidium sp. Laminaria digitata Laminaria saccorhina Phyllophora pseudoceranoides Pilayella littoralis Polysiphonia harveyi Polysiphonia nigrescens Polysiphonia sp. Polysiphonia urceolata Rhodymenia palmata Saccorhiza dermatodea Spermothamnion repens Spermothamnion sp. Trailliella intricata Ulva lactuca |
Anomia simplex Arctica islandica Astarte subaequilatera Astarte undata Clinocardium ciliatum Crassostrea virginica Crenella glandula Gammarus sp. Hiatella arctica Macoma tenta Mercenaria mercenaria Modiolus modiolus Modiolus sp. Mulina lateralis Mya arenia Mya truncata Mytilus edulis Mytilus sp. Nucula proxima Nucula tenuis Pandora glacialis Periploma leanum Petricola pholadiformis Pitar morrhuana Placopecten magellanicus Spisula solidissima Teredo navalis Thyasira gouldii Volsella modiolus Yoldia limatula Yoldia sapotilla Yoldia thraciaeformis |
Acmaea testudinalis Admete couthouyi Aeolidia papillosa Buccinum undatum Coryphella sp. Crepidula convexa Crepidula fornicata Crepidula plana Dendronotus frondosus Eubranchus sp. Facelina bostoniensis Lacuna vincta Littorina sp. Mitrella lunata Nassarius trivittatus Notoacmaea testudinalis Onchidoris Polineces heros Polineces immaculata Urosalpinix cinerea Oenopta (Lora) elegans Oenopta (Lora) turricula |
Aeginella longicornis Balanus balanoides Balanus sp. Cancer irroratus Caprella linearis Caprella sp. Corophium volutator Crangon septemspinosa Homarus americanus Jassa falcata Pagurus acadianus Pagurus pubescens Diastylis quadraspinosa Eudorella trunacta Leucon nasica Eudorella emarginata |
|
Polychaetes
|
Echinoderms
|
||
| Eulalia viridis Eusyllis blomstrandi Gattyana cirrosa Harmothoe sp. Neries sp. Polydora ciliata Phyllodoce sp. |
Asterias forbesi Asterias vulgaris Henricia sp. Ophiura robusta Strongylocentrotus drobachiensis |
||
It is expected that St. Georges Bay will show a large scale fluctuation in biomass through the year; this is a result of the relatively large changes in water temperature with the seasons. Due to growth and metabolism of ectotherms being dependent upon the ambient temperature of the surroundings, it is suggested that biomass will peak during and slightly after temperature maxima and be at a low during the period of temperature minima.