 |
 |
| Correlation Table | Common Splitting | Interpret NMR |
| Give Structure | Next Example | Return to NMR Home |

From the molecular formula, the compound has "1 degree of unsaturation" (one double bond or ring).

Click here for a review on proton NMR.
Click here to view a 1H NMR correlation table.
The proton NMR has two peaks; at high resolution, fine coupling is evident between them (J 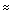 1 Hz) with the peak at
1 Hz) with the peak at  9.7 being a quartet and the peak at
9.7 being a quartet and the peak at  2.1 being a doublet, indicating a CH coupled to a CH3. The peak at
2.1 being a doublet, indicating a CH coupled to a CH3. The peak at  9.7 is in the area generally observed for aldehydic protons; the peak at
9.7 is in the area generally observed for aldehydic protons; the peak at  2.1 is in the region for a methyl group adjacent a carbonyl. The molecule contains oxygen, but the peak at
2.1 is in the region for a methyl group adjacent a carbonyl. The molecule contains oxygen, but the peak at  2.1 is not shifted enough for this to represent an -O- linkage, so it must represent a carbonyl. The very small coupling constant suggests that the proton and the CH3 group are not immediately adjacent, but that this represents an example of cross-space coupling.
2.1 is not shifted enough for this to represent an -O- linkage, so it must represent a carbonyl. The very small coupling constant suggests that the proton and the CH3 group are not immediately adjacent, but that this represents an example of cross-space coupling.
