 |
 |
| Correlation Table | Common Splitting | Interpret NMR |
| Give Structure | Next Example | Return to NMR Home |

From the molecular formula, the compound has "4 degrees of unsaturation" (four double bonds or rings), suggesting the presence of an aromatic compound (benzene has four degrees of unsaturation).

Click here for a review on proton NMR.
Click here to view a 1H NMR correlation table.
The proton NMR has four sets of peaks; a singlet at  3.6 (3H), two sets of doublets centered around
3.6 (3H), two sets of doublets centered around  6.9 (4H), a septet at
6.9 (4H), a septet at  2.7 (1H) and a doublet at
2.7 (1H) and a doublet at  1.6. The singlet at
1.6. The singlet at  3.6 is consistent with an isolated CH3 adjacent to an electronegative center, such as an oxygen. The septet and doublet strongly suggest an isopropyl group
3.6 is consistent with an isolated CH3 adjacent to an electronegative center, such as an oxygen. The septet and doublet strongly suggest an isopropyl group 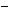 CH(CH3) 2 in which the carbon is bonded to something mildly electronegative and the two doublets centered at
CH(CH3) 2 in which the carbon is bonded to something mildly electronegative and the two doublets centered at  6.9 strongly suggest a 1,4-disubstituted aromatic compound.
6.9 strongly suggest a 1,4-disubstituted aromatic compound.
