 |
NMR: The Chemical Shift
Use the Back Arrow to return to a spectroscopy problem
 |
Since the magnetic dipole of a given nucleus (µ) is a constant, you might predict that all nuclei of a given type would undergo the spin-flip transition at exactly the same applied frequency in a given magnetic field. Fortunately, in a typical organic molecule this is not the case. This is because the electrons in the molecule have small magnetic fields associated with them and these tend to oppose the applied field, screening the nuclei from the full strength of the applied field. The greater the electron density, the greater this 'shielding' will be, hence nuclei which are in electron rich environments will undergo transition at a higher applied field than nuclei in electron poor environments.
The resulting shift in the NMR signal for a given nuclei is referred to as the chemical shift, and, in general, protons or carbons adjacent to electronegative atoms will be deshielded and moved to a higher chemical shift (undergo transition at a lower applied field). The scale utilized for measuring chemical shifts is defined by the equation shown below:
 ) = (shift observed/oscillator frequency) x 106
) = (shift observed/oscillator frequency) x 106 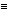 ppm
ppm
The factor of 106 is introduced into the equation to give a simple whole number scale for convenience.
Experimentally, for both 1H and 13C NMR, the  scale is anchored at zero by the NMR absorption of the molecule tetramethyl silane ((CH3)4Si) in which the carbons and protons are more highly shielded than those observed in most common organic molecules. For 1H NMR, the
scale is anchored at zero by the NMR absorption of the molecule tetramethyl silane ((CH3)4Si) in which the carbons and protons are more highly shielded than those observed in most common organic molecules. For 1H NMR, the  scale generally extends from 0-12 ppm; the
scale generally extends from 0-12 ppm; the  scale for 13C nuclei, however, is much larger and covers the range 0-220 ppm.
scale for 13C nuclei, however, is much larger and covers the range 0-220 ppm.
On the  scale for 1H NMR, simple hydrocarbon protons tend to absorb in the region
scale for 1H NMR, simple hydrocarbon protons tend to absorb in the region  0.5-1.5, protons on a carbon adjacent to a carbonyl are shifted to
0.5-1.5, protons on a carbon adjacent to a carbonyl are shifted to 
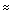 2, electronegative atoms (oxygen or halogens) move
2, electronegative atoms (oxygen or halogens) move  -protons to
-protons to 
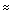 3-4, alkene protons are shifted to
3-4, alkene protons are shifted to 
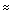 5-6, aromatic protons to
5-6, aromatic protons to 
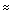 7-8, aldehydic protons to
7-8, aldehydic protons to 
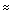 10, and the most highly shifted protons are generally those of carboxylic acids, with values of
10, and the most highly shifted protons are generally those of carboxylic acids, with values of 
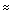 12. Click here to see a table showing these correlations.
12. Click here to see a table showing these correlations.
For 13C NMR, simple methyl carbons tend to absorb in the region  15-30, simple methylene carbons are shifted to
15-30, simple methylene carbons are shifted to 
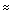 20-65, electronegative atoms (oxygen or halogens) move attached carbons to
20-65, electronegative atoms (oxygen or halogens) move attached carbons to 
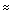 40-80, alkyne carbons are shifted to
40-80, alkyne carbons are shifted to 
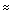 70-90, alkene carbons to
70-90, alkene carbons to 
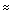 100-150, aromatic carbons to
100-150, aromatic carbons to 
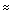 120-170, and the most highly shifted carbons are generally those of carbonyls, with values of
120-170, and the most highly shifted carbons are generally those of carbonyls, with values of 
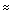 180-220. Click here to see a table showing these correlations.
180-220. Click here to see a table showing these correlations.
One further feature of the proton NMR is the fact that the intensity of the absorbance of a given class of nuclei (with a certain chemical shift) is proportional to the number of protons giving rise to the signal; that is, the area under a given peak (the integration) is directly proportional to the number of that type of proton in the molecule. Integrations are typically given as simplest whole-number ratios, hence, acetic acid, CH3COOH, will have two peaks in the proton NMR, one at  = 2, area = 3, and a second at
= 2, area = 3, and a second at  =12, area = 1. Methyl acetate, CH3COOCH3, will also have two peaks in the proton NMR, one at
=12, area = 1. Methyl acetate, CH3COOCH3, will also have two peaks in the proton NMR, one at  = 2, area = 1, and a second at
= 2, area = 1, and a second at  = 4, area = 1 (the relative areas or both peaks are the same, but each one represents three hydrogens).
= 4, area = 1 (the relative areas or both peaks are the same, but each one represents three hydrogens).
Next Section: Spin Coupling/Splitting
Back to Previous Section