 |
Mass Spectroscopy, Example #5
Analysis: C5H8O2 MW = 100.12
Help with Analysis
Mass Spectrum
| Correlation Table | Common Fragments | Interpret MS |
| Give Structure | Return to MS Home |
 |
Mass Spectroscopy, Example #5
Analysis: C5H8O2 MW = 100.12
Help with Analysis
| Correlation Table | Common Fragments | Interpret MS |
| Give Structure | Return to MS Home |

From the molecular formula, the compound has "2 degrees of unsaturation" (two double bonds, carbonyls or rings).

Fragmentation of Common Functional Groups
The spectrum shows a small molecular ion peak, and a pair of peaks at m/e = 57 and 58. The peak at m/e = 57 corresponds to loss of m/e = 43, which is the base peak and corresponds to the acylium ion (CH3C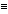 O+). The m/e = 57 fragment corresponds to C3H5O, suggesting the original compound was an ester with this molecular formula.
O+). The m/e = 57 fragment corresponds to C3H5O, suggesting the original compound was an ester with this molecular formula.
Common Ions and Fragments
