 |
 |

From the molecular formula, the compound has "two degrees of unsaturation" (two double bonds, carbonyls or rings).

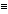 O). The spectrum is consistent with a molecule which can lose methyl or ethoxy radicals, or can undergo fragmentation to form the acylium radical cation.
O). The spectrum is consistent with a molecule which can lose methyl or ethoxy radicals, or can undergo fragmentation to form the acylium radical cation.Click here for a review of mass spectroscopy.
Click here to view a table of common fragments.

Click here for a review of 13C spectroscopy.
Click here to view a 13C correlation table.
The 13C spectrum contains six peaks, indicating that all carbons are unique. The quartets at  14 and 24 represent relatively simple methyl groups; the triplets at
14 and 24 represent relatively simple methyl groups; the triplets at  59 and 47 represent a CH2 groups bonded to mildly electronegative groups; the singlets at
59 and 47 represent a CH2 groups bonded to mildly electronegative groups; the singlets at  207 and 172 are in the carbonyl region, and most likely a ketone or aldehyde (
207 and 172 are in the carbonyl region, and most likely a ketone or aldehyde ( 207) and an ester (
207) and an ester ( 172).
172).

Click here for a review on proton NMR.
Click here to view a 1H-NMR correlation table.
Click here for a review on Infrared Spectroscopy.
Click here to view an IR correlation table.
The mass spectrum displays a molecular ion and the base peak represents the formation of the acylium ion, indicating the presence of a methyl adjacent to a carbonyl. The presence of an m-45 peak strongly suggests the presence of an ethoxy group.
The 13C spectrum contains six peaks, indicating that all carbons are unique. The quartets at
The proton NMR shows evidence for an ethyl group and isolated CH2 and CH3 groups. The methylene of the ethyl group must be next to an electronegative atom (most likely oxygen) suggesting an -OCH2CH3 group. The isolated CH2 must also be flanked by mildly electronegative groups, and the isolated CH3 is in the region often observed for methyls adjacent to carbonyls.
The IR is consistent with a simple saturated hydrocarbon, possibly containing two carbonyls (based on the side peak at
The simplest structure which is consistent with all of these data would be a dicarbonyl compound containing an ethoxy residue and a methyl ketone (based on the presence of the acylium ion in the MS).
The proton NMR has a quartet coupled to a triplet, indicative of an ethyl group. The CH2 must be adjacent to an electron withdrawing group since it is shifted to  4.1. The two singlets at
4.1. The two singlets at  2.2 and 3.2 suggest isolated CH2 and CH3 groups and the CH2 must be adjacent to one or more electronegative groups.
2.2 and 3.2 suggest isolated CH2 and CH3 groups and the CH2 must be adjacent to one or more electronegative groups.
3400-3200 cm-1: no OH peak (too small)
3100 cm-1: no significant peak, suggesting no unsaturated CH
2900 cm-1: strong peak suggesting saturated CH
2200 cm-1: no unsymmetrical triple bonds
1710 cm-1: strong carbonyl with a second peak at 1670 cm-1, suggesting a
the possibility of two carbonyls
1600 cm-1: no significant peaks, suggesting no carbon-carbon double bonds
The molecule contains an oxygen, and from the analysis, contains two double bonds, carbonyls or rings. 14 and 24 represent relatively simple methyl groups; the triplets at
14 and 24 represent relatively simple methyl groups; the triplets at  59 and 47 represent a CH2 groups bonded to mildly electronegative groups; the singlets at
59 and 47 represent a CH2 groups bonded to mildly electronegative groups; the singlets at  207 and 172 are in the carbonyl region, and most likely a ketone or aldehyde (
207 and 172 are in the carbonyl region, and most likely a ketone or aldehyde ( 207) and an ester (
207) and an ester ( 172).
172). 1670 cm-1). The minor peak at 3400 cm-1 is too small to be an -OH.
1670 cm-1). The minor peak at 3400 cm-1 is too small to be an -OH.